|
Reaction Mechanisms
|
||||||
|
|
||||||
 |
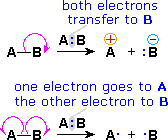 |
|
 |
![]() The use of these symbols in bond-breaking and bond-making reactions is illustrated below. If a covalent single bond is broken so that one electron of the shared pair remains with each fragment, as in the first example, this bond-breaking is called homolysis.
The use of these symbols in bond-breaking and bond-making reactions is illustrated below. If a covalent single bond is broken so that one electron of the shared pair remains with each fragment, as in the first example, this bond-breaking is called homolysis.
![]() If the bond breaks with both electrons of the shared pair remaining with one fragment, as in the second and third examples, this is called heterolysis.
If the bond breaks with both electrons of the shared pair remaining with one fragment, as in the second and third examples, this is called heterolysis.

Other Arrow Symbols
![]() Chemists also use arrow symbols for other purposes, and it is essential to use them correctly.
Chemists also use arrow symbols for other purposes, and it is essential to use them correctly.


 2. Reactive Intermediates
2. Reactive Intermediates
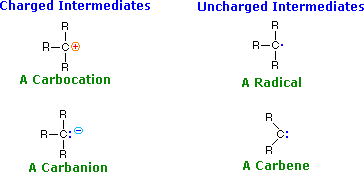
![]() The products of bond breaking, shown above, are not stable in the usual sense, and cannot be isolated for prolonged study. Such species are referred to as reactive intermediates, and are believed to be transient intermediates in many reactions. The general structures and names of four such intermediates are given below.
The products of bond breaking, shown above, are not stable in the usual sense, and cannot be isolated for prolonged study. Such species are referred to as reactive intermediates, and are believed to be transient intermediates in many reactions. The general structures and names of four such intermediates are given below.
![]() A pair of widely used terms, related to the Lewis acid-base notation, should also be introduced here.
A pair of widely used terms, related to the Lewis acid-base notation, should also be introduced here.
![]() Using these definitions, it is clear that carbocations ( called carbonium ions in the older literature ) are electrophiles and carbanions are nucleophiles. Carbenes have only a valence shell sextet of electrons and are therefore electron deficient. In this sense they are electrophiles, but the non-bonding electron pair also gives carbenes nucleophilic character. As a rule, the electrophilic character dominates carbene reactivity.
Using these definitions, it is clear that carbocations ( called carbonium ions in the older literature ) are electrophiles and carbanions are nucleophiles. Carbenes have only a valence shell sextet of electrons and are therefore electron deficient. In this sense they are electrophiles, but the non-bonding electron pair also gives carbenes nucleophilic character. As a rule, the electrophilic character dominates carbene reactivity.
![]() Carbon radicals have only seven valence electrons, and may be considered electron deficient; however, they do not in general bond to nucleophilic electron pairs, so their chemistry exhibits unique differences from that of conventional electrophiles. Radical intermediates are often called free radicals.
Carbon radicals have only seven valence electrons, and may be considered electron deficient; however, they do not in general bond to nucleophilic electron pairs, so their chemistry exhibits unique differences from that of conventional electrophiles. Radical intermediates are often called free radicals.
![]() The importance of electrophile / nucleophile terminology comes from the fact that many organic reactions involve at some stage the bonding of a nucleophile to an electrophile, a process that generally leads to a stable intermediate or product. Reactions of this kind are sometimes called ionic reactions, since ionic reactants or products are often involved.
The importance of electrophile / nucleophile terminology comes from the fact that many organic reactions involve at some stage the bonding of a nucleophile to an electrophile, a process that generally leads to a stable intermediate or product. Reactions of this kind are sometimes called ionic reactions, since ionic reactants or products are often involved.
Virtual Textbookの目次へ